Entradas
Mostrando las entradas de 2016
| Lista de correo. Espere su aprobación. |
| Consultar este grupo |
#HISTORY: The Spanish royal philanthropic expedition to bring #smallpox vaccination to the New World, XIX Century
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Use of transgenic Aedes aegypti in Brazil: risk perception and assessment.
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Película completa "22 angeles" online, por RTVE. Hasta Dic 27, 2016. #viruela
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Revista Mexicana de Bioseguridad 2016
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Establishing protocols for tick containment at Biosafety Level 4.
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Preparation of viral samples within biocontainment for ultrastructural analysis
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
#VIDEO: Safety Precautions and Operating Procedures in an (A)BSL-4 Laboratory
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Containment of Arthropod Disease Vectors
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Communicable Diseases and Outbreak Control
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Informe Mundial sobre el Paludismo 2015
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Recent Advances in Antimicrobial Polymers: A Mini-Review
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
VIDEO: Safety procedures for handling sharps
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
The Weekly Epidemiological Record (WER)
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Niveles de bioseguridad
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
#WEBINAR: The Necrobiome - Microbial Life After Death
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
OCTUBRE 15: Día mundial del lavado de manos "Siempre lave sus manos"
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
HIGH-CONTAINMENT LABS: Improved Oversight of Dangerous Pathogens Needed to Mitigate Risk
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Measuring Pathogen Decay in Bioaerosols
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Concentrations and Sources of Airborne Particles in a Neonatal Intensive Care Unit
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Recent advances in synthetic biosafety
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
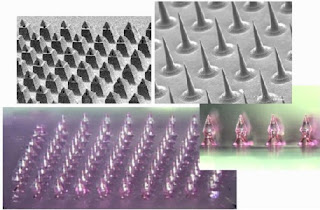
Microneedles: A New Frontier in Nanomedicine Delivery
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
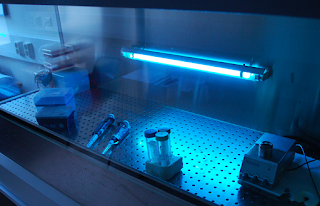
Use of Ultraviolet (UV) Lights in Biological Safety Cabinets
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Respiratory Health in Waste Collection and Disposal Workers
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
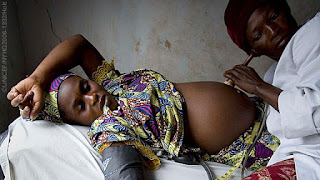
Pregnancy, Labor, Delivery & Ebola: Implications for Infection Control in Obstetrics
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
#Ebola response in Sierra Leone: The impact on children
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Quantitative Microbial Risk Assessment in Occupational Settings Applied to the Airborne Human Adenovirus Infection
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Occupational health related concerns among surgeons
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Prevalence of Respiratory Protective Devices in U.S. Health Care Facilities
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Indications and the requirements for single-use medical gloves
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Engineered nanomaterials: toward effective safety management in research laboratories
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
#UANL: 2º Taller "Control de Riesgos Biológicos en Laboratorios de Investigación"
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Use of Protective Gloves in Nail Salons in Manhattan, New York City
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Performance analysis of exam gloves used for aseptic rodent surgery
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Gain-of-Function Research: Ethical Analysis
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Soliciting Stakeholder Input for a Revision of Biosafety in Microbiological and Biomedical Laboratories (BMBL): Proceedings of a Workshop.
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Interim Guidance for Health Care Providers Caring for Pregnant Women with Possible #Zika Virus Exposure
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
HISTORY 1951: Yellow fever and Max Theiler: the only Nobel Prize for a virus vaccine
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
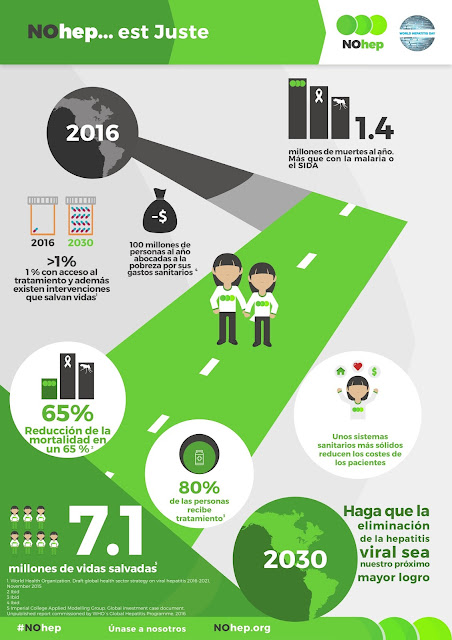
Día Mundial contra la Hepatitis, Julio 28 / #Hepatitis world day, July 28th #NoHep
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Hipoclorito de sodio como agente desinfectante
- Obtener vínculo
- X
- Correo electrónico
- Otras apps