Entradas
Mostrando las entradas de septiembre, 2017
| Lista de correo. Espere su aprobación. |
| Consultar este grupo |
Herramientas para la gestión del riesgo químico
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Recomendaciones del @CDC en caso de terremotos. #fuertemexico #fuerzamexico
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Unintended spread of a #biosafety level 2 recombinant retrovirus
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
MAPA Información Centros de Acopio, derrumbes, hospitales y voluntariado #FuerzaMexico
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
#WebinarAMEXBIO Transporte de Sustancias Infecciosas entre Instituciones
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Detection of viral proteins in human cells lines by xeno-proteomics
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Selecting Protective Clothing for Protection against Microorganisms in Blood and Body Fluids
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Guidance for Donning and Doffing Personal Protective Equipment (PPE) for #Ebola
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
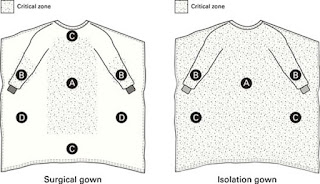
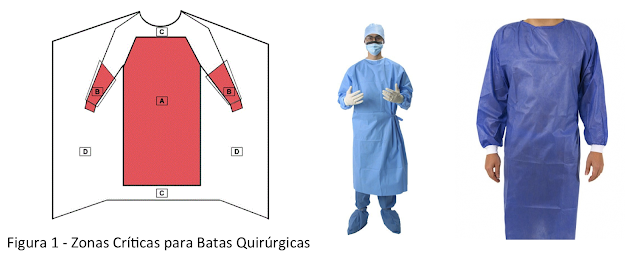
Acerca de las batas médicas
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Todo lo que necesita saber sobre el uso de respiradores #N95Day
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
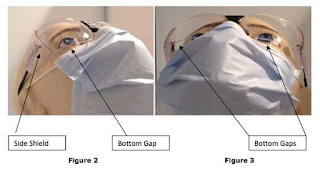
Eye Safety in Dentistry and Associated Liability
- Obtener vínculo
- X
- Correo electrónico
- Otras apps