Entradas
Mostrando las entradas de octubre, 2018
| Lista de correo. Espere su aprobación. |
| Consultar este grupo |
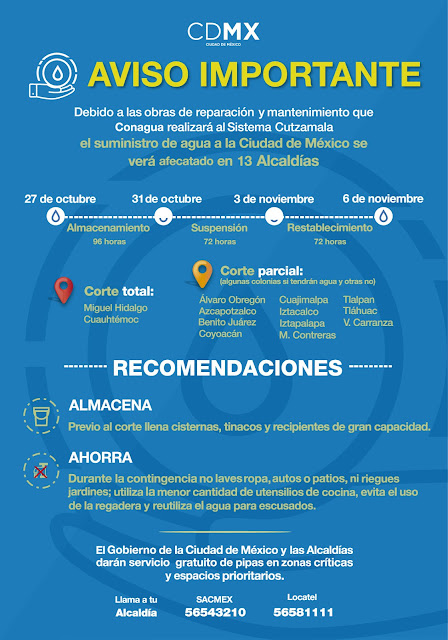
Corte de Agua 2018
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
#WebinarAMEXBIO: Conceptos básico sobre residuos peligrosos biológico infecciosos (RPBI's)
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Membresías AMEXBIO 2019
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Promoting Scientific Transparency to Facilitate the Safe and Open International Exchange of Biological Materials and Electronic Data
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Bioterrorism and the Role of the Clinical Microbiology Laboratory
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Synthetic viruses-Anything new?
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
A mobile biosafety microanalysis system for infectious agents
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Smart Card Decontamination in a High-Containment Laboratory
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Biosafety controls come under fire
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Laboratory-Acquired Vaccinia Virus Infection in a Recently Immunized Person — Massachusetts, 2013
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Laboratory-associated infections and biosafety.
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Evidence-Based Biosafety
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Antibiotics, #Resistome and Resistance Mechanisms: A Bacterial Perspective
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Cabinas de seguridad biológica: Uso, desinfección y mantenimiento
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
The basics of animal biosafety and biocontainment training
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Chemical Use in Animal Models
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Zoonotic Infections from Hantavirus and Lymphocytic Choriomeningitis Virus (LCMV) Associated with Rodent Colonies That Were Not Experimentally Infected
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Animal Research Biosafety
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Institutional Responsibilities for the Oversight of Personnel Safety in Animal Research
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
High Containment Pathogen Preparation in the Intensive Care Unit
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
A Review of Laboratory-Acquired Infections in the Asia-Pacific
- Obtener vínculo
- X
- Correo electrónico
- Otras apps