Entradas
Mostrando las entradas de noviembre, 2017
| Lista de correo. Espere su aprobación. |
| Consultar este grupo |
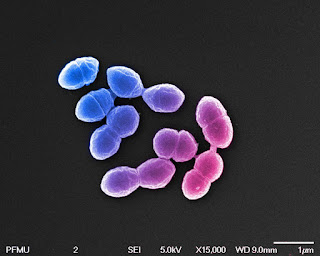
Enterococcus hirae biofilm formation on hospital material surfaces and effect of new biocides
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Guidance on regulations for the transport of infectious substances 2017–2018
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Biological samples transportation by drones: ready for prime time?
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Molecular Viability Testing of UV-Inactivated Bacteria
- Obtener vínculo
- X
- Correo electrónico
- Otras apps