Entradas
Mostrando las entradas de septiembre, 2015
| Lista de correo. Espere su aprobación. |
| Consultar este grupo |
Política gubernamental para la investigación de preocupación de uso dual #DURC
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
CDC. Workbook for Designing, Implementing and Evaluating a Sharps Injury Prevention Program
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
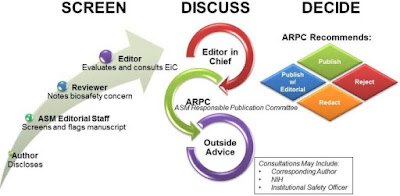
Dual-Use Research of Concern (#DURC) Review at American Society for Microbiology Journals
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Novel Human Virus That Shares Genomic Features with Hepaciviruses and Pegiviruses
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
State-of-the-Art in Biosafety and Biosecurity in European Countries
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
To PAPR or not to PAPR?
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Health worker #Ebola infections in Guinea, Liberia and Sierra Leone
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Genetic Analysis of a Sarcoma Accidentally Transplanted from a Patient to a Surgeon #LAIs
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Reduced Efficiency of Chlorine Disinfection of Naegleria fowleri in a Drinking Water Distribution Biofilm
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
WHO Guidelines for Containment of Poliovirus Following Type-Specific #Polio Eradication
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
MERS-CoV in Upper Respiratory Tract and Lungs of Dromedary Camels, Saudi Arabia, 2013-2014
- Obtener vínculo
- X
- Correo electrónico
- Otras apps