Entradas
Mostrando las entradas de 2017
| Lista de correo. Espere su aprobación. |
| Consultar este grupo |
NOM-003-SEGOB-2011, Señales y avisos para protección civil.- Colores, formas y símbolos a utilizar.
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Lessons to be Learned from #Biosafety Incidents in the USA
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Enhancing Surveillance and Diagnostics in Anthrax-Endemic Countries
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Shelf-Life of Chlorine Solutions Recommended in Ebola Virus Disease Response
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Enterococcus hirae biofilm formation on hospital material surfaces and effect of new biocides
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Guidance on regulations for the transport of infectious substances 2017–2018
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Biological samples transportation by drones: ready for prime time?
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Molecular Viability Testing of UV-Inactivated Bacteria
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
#History 1990: Model for inactivation and disposal of infectious HIV and radioactive waste in a BL3 facility
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Dead or Alive: Molecular Assessment of Microbial Viability
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Schrödinger’s microbes: Tools for distinguishing the living from the dead in microbial ecosystems
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Workplace Hazards to Reproduction and Development
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Laboratory-acquired infections of Salmonella enterica serotype Typhi in South Africa
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Prevalence of murine leukemia virus contamination in human cell lines
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Día mundial del lavado de manos, Octubre 15, 2017
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Triage and Management of Accidental Laboratory Exposures to Biosafety Level-3 and -4 Agents
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
La gestión de cadáveres en situaciones de desastre #bioseguridad
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Virus detection in Human and Other Primate Cell Lines
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
The Effects of Workplace Hazards on Female Reproductive Health
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Hospital Decontamination Self-Assessment Tool
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Best Practices for Hospital-Based First Receivers
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
INFECTION RISKS to new and expectant mothers in the workplace
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Herramientas para la gestión del riesgo químico
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Recomendaciones del @CDC en caso de terremotos. #fuertemexico #fuerzamexico
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Unintended spread of a #biosafety level 2 recombinant retrovirus
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
MAPA Información Centros de Acopio, derrumbes, hospitales y voluntariado #FuerzaMexico
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
#WebinarAMEXBIO Transporte de Sustancias Infecciosas entre Instituciones
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Detection of viral proteins in human cells lines by xeno-proteomics
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Selecting Protective Clothing for Protection against Microorganisms in Blood and Body Fluids
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Guidance for Donning and Doffing Personal Protective Equipment (PPE) for #Ebola
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
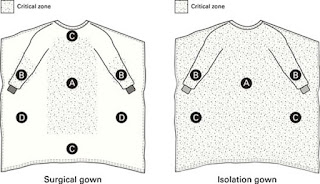
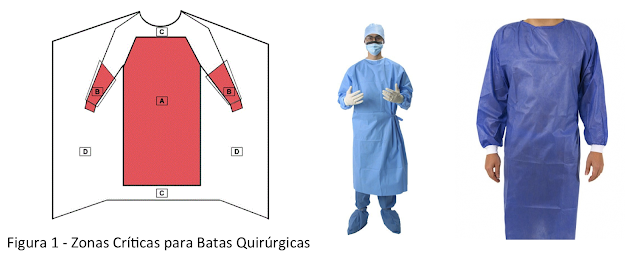
Acerca de las batas médicas
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
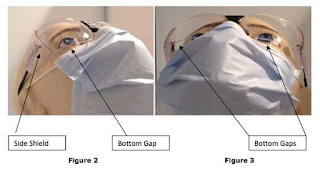
Todo lo que necesita saber sobre el uso de respiradores #N95Day
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Eye Safety in Dentistry and Associated Liability
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Cost-effectiveness analysis of N95 respirators and medical masks to protect healthcare workers in China
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
Respirator Performance against Nanoparticles under Simulated Workplace Activities
- Obtener vínculo
- X
- Correo electrónico
- Otras apps
#WEBINAR: Contención biológica: barreras primarias y secundarias
- Obtener vínculo
- X
- Correo electrónico
- Otras apps